density molarity formula|molarity calculator using density : Tagatay By convention, the concentration in molarity of a given compound is often referred to using the formula of the compound within square brackets; thus [HCOOH] means "the molarity of formic acid" in a given context, e.g., . Trial Xtreme 2 is the sequel to multi-million selling hit Trial Xtreme, packed with more levels, amazing new graphics and more blistering motorcycle stunt action than ever. Crank up the throttle, rev your engine and negotiate your way across 42 new action-packed levels.
0 · molarity formula with answers
1 · molarity calculator using density
2 · molarity calculation examples
3 · molar concentration formula with density
4 · formula of molarity terms density
5 · formula of molarity class 11
6 · density to molarity calculator
7 · calculate density from concentration
8 · More
SKU: 587. R$ 9.899,00. Elevador tipo escada para elevação de painéis solares. Segurança e praticidade, equipamento muito versátil, de fácil e rápida montagem/desmontagem. Elevador para Painel Solar Até 20 .
density molarity formula*******Molarity-to-Density Formula. Molar concentration, also called molarity, amount concentration or substance concentration, is a measure of the concentration of a solute . By definition, molarity c c is the amount of substance n n per volume of solution V V: c = n V (1) (1) c = n V. The volume V V can be found using total mass of .
Convert the expressions above to obtain a molarity formula. As mass/volume = molarity × molar mass, then mass / (volume × molar mass) = molarity. .By convention, the concentration in molarity of a given compound is often referred to using the formula of the compound within square brackets; thus [HCOOH] means "the molarity of formic acid" in a given context, e.g., .
Molarity Equation. As shown below, the molarity of a solution is defined as the ratio of the molar amount of solute that is present in a solution, relative to the .density molarity formula molarity calculator using density The molar mass of CoCl 2 •2H 2 O is 165.87 g/mol. Therefore, molesCoCl2 ⋅ 2H2O = ( 10.0g 165.87g / mol) = 0.0603mol. The volume of the solution in liters is. .
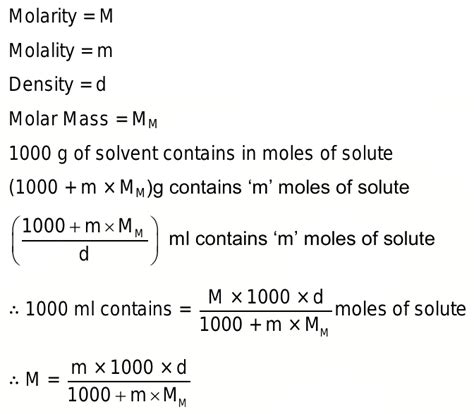
molarity calculator using densityCalculate molality of 1M HCl solution having density 1.5365g/mol. Q. For a solution of density, d in g/ml containing solute of molecular weight W, the molarity M and molality m are related by: Q. If M is the molarity, d is .
Molarity. The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution. A solution that is 1.00 molar (written .
density molarity formula Molarity. The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution. A solution that is 1.00 molar (written .When performing calculations stepwise, as in Example 3.17, it is important to refrain from rounding any intermediate calculation results, which can lead to rounding errors in the .

Practice questions on molarity and molality: 15.0 g of NaOH is dissolved in enough water to make a total of 224 mL of solution. Calculate the molarity. Given, 123.2 gm of NaOH is dissolved in 1 kg of water. Calculate the molality. To learn more about molarity and molality from the experts register now! Other important links:
3. Divide the number of moles by the number of liters. The resulting quotient will give you the number of moles per liter of solution, otherwise known as molarity. [3] Example problem: molarity = moles of .
Solution. Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Per this definition, the solution volume must be converted from mL to L: M = molsolute Lsolution = 0.133mol 355mL × 1L 1000mL = 0.375M. This video explains how to calculate the concentration of the solution in forms such as Molarity, Molality, Volume Percent, Mass Percent, and Mole Fraction. .Molarity-to-Density Formula. Molar concentration, also called molarity, amount concentration or substance concentration, is a measure of the concentration of a solute in a solution, or of any chemical species, in terms of the amount of substance in a given volume.
Molarity. The most common way to express solution concentration is molarity (M), which is defined as the amount of solute in moles divided by the volume of solution in liters: M = moles of solute/liters of solution. A solution that is 1.00 molar (written 1.00 M) contains 1.00 mole of solute for every liter of solution.Created by Sal Khan.
After entering the customized values of the selected acids or bases; % purity, density, formula weight, desired volume, and desired concentration, you can easily determine the required volume to make the desired solution. For example, the % purity of the concentrated nitric acid is 70 %. Its density is 1.413 g/mL and its formula weight is .Example #1: Given a density of 1.836 g/mL and a mass percent of H 2 SO 4 of 96.00%, find the molarity, molality, and mole fraction. The molar mass of water is 18.015 g/mol and the molar mass of sulfuric acid is 98.078 g/mol. (Two different starting assumptions are shown.) Solution assuming a certain volume of solution is present:
Plug the number of moles and the mass of the solvent into the molality formula. Divide 1.2 mol by 1.5 kg, and you'll find out that the molality of the NaCl solution is 0.8 molal (in standard molality units: 0.8 mol/kg). Or save yourself some time and use our molality calculator (choose advanced mode to enter also the molar mass and solute .
By convention, the concentration in molarity of a given compound is often referred to using the formula of the compound within square brackets; thus [HCOOH] means "the molarity of formic acid" in a given context, e.g., when its concentration is 0.500 mol/L (or 0.500 molar), it would be written as [HCOOH] = 0.500 M.
According to the definition of molarity, the molar amount of solute in a solution is equal to the product of the solution’s molarity and its volume in liters: n = ML (6.5.17) (6.5.17) n = M L. Expressions like these may be written for a solution before and after it is diluted: n1 = M1L1 (6.5.18) (6.5.18) n 1 = M 1 L 1.Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution. In chemistry, the most commonly used unit for molarity is the number of moles per liter, .
Resultado da Onlyflix Play - Login. . Login. Resetar a Senha Lembrar de mim. Não tem uma conta?
density molarity formula|molarity calculator using density